Friday, December 16, 2016
Bioenergy Research Group
http://www2.hawaii.edu/~khanal/fungal/fungalfermentation.html
Fungal Fermentation as a Value-added Processing of Sugarcane-Ethanol Vinasse
Vinasse has high organic content especially protein from yeast cell leftover which can be served as an excellent source of nutrients to support fungal growth. Fungi can produce several extracellular enzymes thereby facilitating the degradation of recalcitrant compounds in vinasse. Filamentous fungi require simple process for fungal biomass separation consequently fungal biomass production cost could be relatively low. In general, because fungal biomass has relatively high protein content around 50% (on dry wt. basis), the main purpose of fungal process is for producing single cell protein (SCP) for food/feed applications.
Rhizopus microsporus var. oligosporus (shortened as R. oligosporus) is an edible filamentous fungal species.

Some important characteristics of R. oligosporus are:
Food-grade filamentous fungus: R. oligosporus is known as a starting culture for making “tempeh” an Indonesian delicacy.
FDA-approved fungal protein: R. oligosporus has been classified as a biosafety level 1 (BSL-1), which is not known to cause disease in immumocompetent adult human. It is not associated with the production of any potentially harmful metabolites. Also, the fungus has been given generally recognized as safe (GRAS) status by the U.S. Food and Drug Administration (FDA). Therefore, the fungus produces food-grade fungal biomass with high protein content.

Our research aims to study fungal technology as an environment-friendly approach on utilizing sugarcane-ethanol derived vinasse. We optimize fungal growth conditions on vinasse in 250-ml Erlenmeyer flask containing 100 ml vinasse. Under optimal fungal growth condition, we examine fungal fermentation on vinasse in two bioreactor configurations (airlift bioreactor and bubble column bioreactor) with 2.5 L working volume. It is important to note that, because of lack of existing sugarcane ethanol facility in the US, vinasse used in our study has been prepared in our laboratory through yeast fermentation of sugarcane juice and sugarcane syrup obtained from Hawaiian Commercial & Sugar Company (HC&S) (Puunene, HI, USA). We also examined fungal biomass quality for animal feed application.
We first investigated if the fungus could utilize vinasse to support its growth with concomitant water reclamation. Growth conditions, including vinasse concentration, pH, nutrient supplementation, and sample sterilization, were optimized in a sequential order as shown in Fig. 3. We determined fungal biomass (dry weight) and organic matter removal as soluble chemical oxygen demand (SCOD) as efficacy of fungal process.
The optimal fungal growth conditions were undiluted vinasse at a pH of 5.0 with nutrient supplementation. Under the optimal growth conditions, we achieved fungal biomass of 1.12 g biomass increase/g initial biomass with SCOD removal of 49%.

Fungal growth and morphology in submerged fermentation depend on a wide range of parameters, such as cultivation media, agitation intensity, and shear stress. In general, free filamentous mycelia (suspended mycelia), clumps, and pellets are the three major fungal morphologies formed when cultivating filamentous fungi in liquid media. Our research aims at producing fungal biomass in pellets form because it has less adverse effect on rheological properties of the fermentation broth. We fabricated our reactors by eliminating mechanical mixing. In our reactor design, the air was supplied at the bottom of the reactors that provided sufficient mixing for the fungal fermentation. We also studied the effect of fungal growth on two bioreactor configurations, airlift and bubble column, and the impact of various aeration rates (0.5, 1.0, 1.5, and 2.0 volume air/volume liq/min (vvm)) on fungal biomass production.
Ethanol fermentation was carried out in our laboratory by the yeast, S. cerevisiae, fermentation of sugarcane syrup. After ethanol recovery using rotary evaporator, vinasse was kept refrigerated until further use.

Prior to fungal fermentation in bioreactor, we precultured the fungus in 1 L Erlenmeyer flask containing 500-ml yeast mold (YM) broth. Fungal spores are derived from rehydrated and reactivated freeze-dried fungal culture obtained from the American Type Culture Collection (ATCC # 22959, Rockville, MD).

Internal loop bioreactors with 3.5-L total volume were fabricated in our laboratory facility (Fig. 6). Airlift reactors were made of clear acrylic plastic with the removable draft tube providing the ratio of the downcomer and riser cross-sectional area (Ad/Ar) of about 1. Air was supplied through simple porous air diffusers at the bottom of the riser section. The airlift reactors were used without draft tube to conduct experiments on fungal cultivation on vinasse in the bubble column reactor
Aeration rate in fungal cultivation directly affected fungal biomass production in bioreactors. The optimal aeration in fungal fermentation in both airlift and bubble column reactors was 1.5 vvm. Air bubble column reactor eliminated the problem associated with fungal biomass agglomeration at the annulus section between the draft tube and external column. The effluent after fungal fermentation was in a better quality as the organic content removal was in the range of 75-80% for all samples.


The integration of fungal fermentation in bioethanol facilities is believed to be able to improve the economic viability and sustainability of the ethanol plants. Fungal biomass has a potential for animal feed applications as it contains high protein with nearly all important essential amino acids necessary for animal feeds.


Rhizopus microsporus (var. oligosporus)
Rhizopus microsporus var. oligosporus (shortened as R. oligosporus) is an edible filamentous fungal species.

Fig. 1 R. oligosporus grown on solid media (a) and liquid media (b)
Some important characteristics of R. oligosporus are:

Fig. 2 Tempeh
Our Fungal Fermentation Technology
Our research aims to study fungal technology as an environment-friendly approach on utilizing sugarcane-ethanol derived vinasse. We optimize fungal growth conditions on vinasse in 250-ml Erlenmeyer flask containing 100 ml vinasse. Under optimal fungal growth condition, we examine fungal fermentation on vinasse in two bioreactor configurations (airlift bioreactor and bubble column bioreactor) with 2.5 L working volume. It is important to note that, because of lack of existing sugarcane ethanol facility in the US, vinasse used in our study has been prepared in our laboratory through yeast fermentation of sugarcane juice and sugarcane syrup obtained from Hawaiian Commercial & Sugar Company (HC&S) (Puunene, HI, USA). We also examined fungal biomass quality for animal feed application.
Fungal Growth Optimization Study
We first investigated if the fungus could utilize vinasse to support its growth with concomitant water reclamation. Growth conditions, including vinasse concentration, pH, nutrient supplementation, and sample sterilization, were optimized in a sequential order as shown in Fig. 3. We determined fungal biomass (dry weight) and organic matter removal as soluble chemical oxygen demand (SCOD) as efficacy of fungal process.
The optimal fungal growth conditions were undiluted vinasse at a pH of 5.0 with nutrient supplementation. Under the optimal growth conditions, we achieved fungal biomass of 1.12 g biomass increase/g initial biomass with SCOD removal of 49%.

Fig. 3 Summary of experimental plan for fungal growth optimization studies
Fungal Cultivation in Bioreactor System
Fungal growth and morphology in submerged fermentation depend on a wide range of parameters, such as cultivation media, agitation intensity, and shear stress. In general, free filamentous mycelia (suspended mycelia), clumps, and pellets are the three major fungal morphologies formed when cultivating filamentous fungi in liquid media. Our research aims at producing fungal biomass in pellets form because it has less adverse effect on rheological properties of the fermentation broth. We fabricated our reactors by eliminating mechanical mixing. In our reactor design, the air was supplied at the bottom of the reactors that provided sufficient mixing for the fungal fermentation. We also studied the effect of fungal growth on two bioreactor configurations, airlift and bubble column, and the impact of various aeration rates (0.5, 1.0, 1.5, and 2.0 volume air/volume liq/min (vvm)) on fungal biomass production.
Vinasse Preparation
Ethanol fermentation was carried out in our laboratory by the yeast, S. cerevisiae, fermentation of sugarcane syrup. After ethanol recovery using rotary evaporator, vinasse was kept refrigerated until further use.

Fig. 4 Ethanol fermentation for vinasse preparation (a) and vinasse (b)
Fungal Starter Culture
Prior to fungal fermentation in bioreactor, we precultured the fungus in 1 L Erlenmeyer flask containing 500-ml yeast mold (YM) broth. Fungal spores are derived from rehydrated and reactivated freeze-dried fungal culture obtained from the American Type Culture Collection (ATCC # 22959, Rockville, MD).

Fig. 5 Fungal starter culture
Bioreactors Design
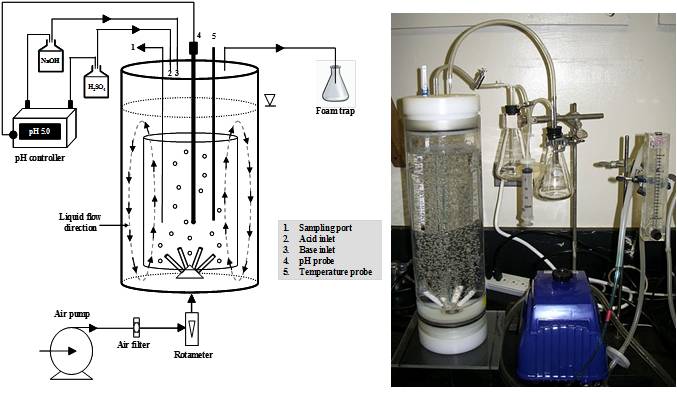
Internal loop bioreactors with 3.5-L total volume were fabricated in our laboratory facility (Fig. 6). Airlift reactors were made of clear acrylic plastic with the removable draft tube providing the ratio of the downcomer and riser cross-sectional area (Ad/Ar) of about 1. Air was supplied through simple porous air diffusers at the bottom of the riser section. The airlift reactors were used without draft tube to conduct experiments on fungal cultivation on vinasse in the bubble column reactor

Fig. 6 An airlift bioreactor system
Fungal Cultivation in Bioreactors at various Aeration Rates
Aeration rate in fungal cultivation directly affected fungal biomass production in bioreactors. The optimal aeration in fungal fermentation in both airlift and bubble column reactors was 1.5 vvm. Air bubble column reactor eliminated the problem associated with fungal biomass agglomeration at the annulus section between the draft tube and external column. The effluent after fungal fermentation was in a better quality as the organic content removal was in the range of 75-80% for all samples.

Fig. 7 Changes in fungal morphologies during fungal fermentation in airlift reactor at 1.5 vvm: 16 h (a); 24 h (b); 48 h (c); and 72 h (d)

Fig. 8 Fungal biomass yields in airlift and bubble column bioreactors at various aeration rates (0.5, 1.0, 1.5, and 2.0 vvm)
Fungal Biomass Characteristics
The integration of fungal fermentation in bioethanol facilities is believed to be able to improve the economic viability and sustainability of the ethanol plants. Fungal biomass has a potential for animal feed applications as it contains high protein with nearly all important essential amino acids necessary for animal feeds.
Table 1 Compositions of vinasse-derived fungal biomass, soybean meal, and fishmeal


Fig. 9 The percentage essential amino acids (% protein basis) of fishmeal, soybean meal, and vinasse-derived fungal biomass)
Subscribe to:
Comments (Atom)









